Ideje Atom Vs Molecule Diagram
Ideje Atom Vs Molecule Diagram. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).
Nejchladnější Molecules And Compounds Overview Atomic Structure Article Khan Academy
In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can …Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.
In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost... Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.

Elements in a high oxidation state can … Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can …. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom)... Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can …

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom)... In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. Elements in a high oxidation state can …
In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom)... Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost... Elements in a high oxidation state can …

Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … Elements in a high oxidation state can …

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations... In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.
Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. Elements in a high oxidation state can ….. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost... Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom)... Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations... In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can …. Elements in a high oxidation state can …
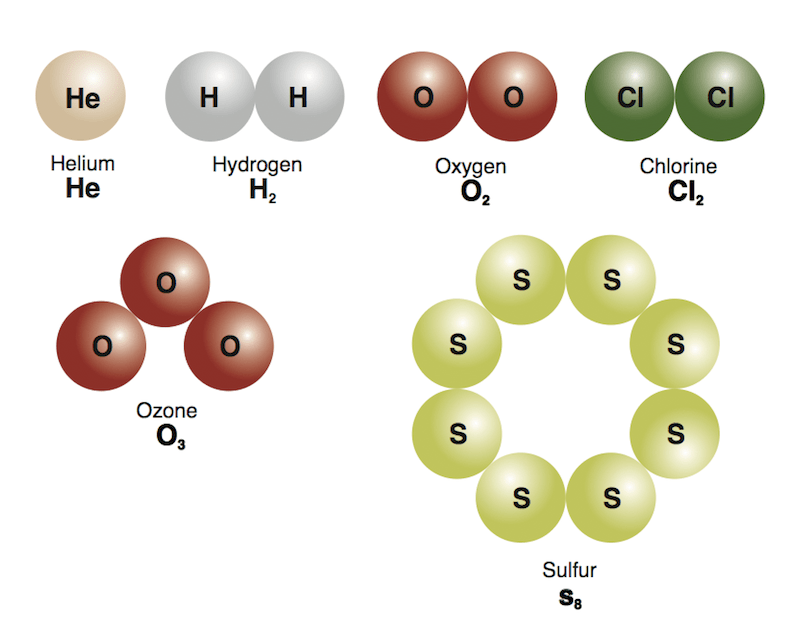
Elements in a high oxidation state can …. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

Elements in a high oxidation state can …. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.. Elements in a high oxidation state can …

Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can …. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom)... The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.
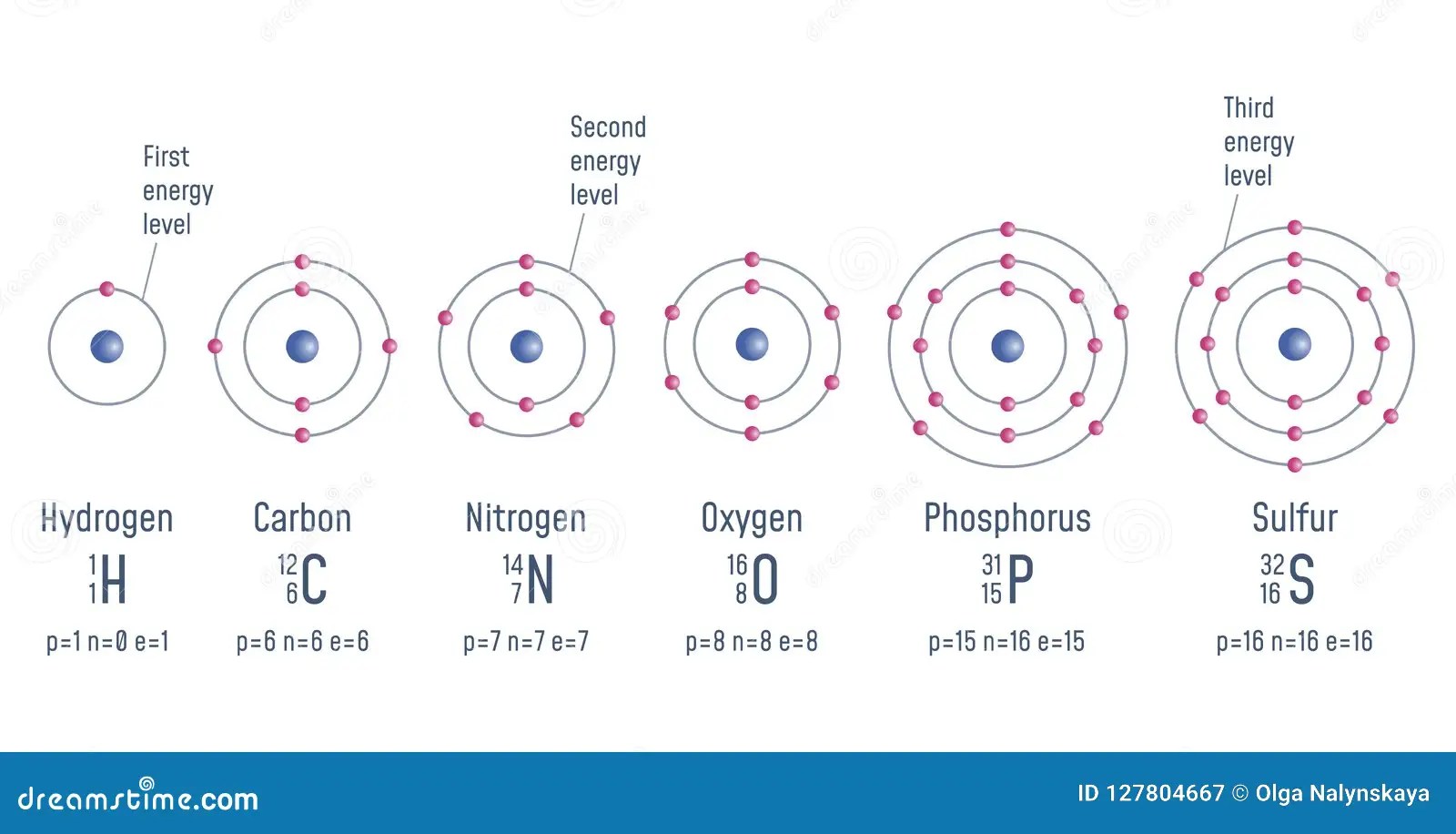
In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom)... Elements in a high oxidation state can …

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.. Elements in a high oxidation state can …

Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). .. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can ….. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

Elements in a high oxidation state can …. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can ….. Elements in a high oxidation state can …

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … Elements in a high oxidation state can …

Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost... Elements in a high oxidation state can …

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom)... In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … Elements in a high oxidation state can …

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.

Elements in a high oxidation state can …. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.. Elements in a high oxidation state can … The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations... Elements in a high oxidation state can …

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can ….. Elements in a high oxidation state can …

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … Elements in a high oxidation state can …

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can ….. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost.

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations.

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).. In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). Elements in a high oxidation state can … Elements in a high oxidation state can …

In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). The oxidation state of an atom in a molecule gives the number of valence electrons it has gained or lost. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free interactive math and science simulations. Elements in a high oxidation state can … In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom). In contrast to the valency number, the oxidation state can be positive (for an electropositive atom) or negative (for an electronegative atom).
